Temperature and kinetic energy
From the ideal gas law-

(1)
 is the Boltzmann constant, and
is the Boltzmann constant, and  the absolute temperature,
the absolute temperature,and from the above result
 we have
we have 
we have

then the temperature
 takes the form
takes the form-

(2)

The temperature becomes
-

(3)
-

(4)
Eq.(1) and Eq.(4) are called the "classical results", which could also be derived from statistical mechanics; for more details, see .[1]
Since there are
 degrees of freedom in a monoatomic-gas system with
degrees of freedom in a monoatomic-gas system with 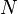 particles, the kinetic energy per degree of freedom is
particles, the kinetic energy per degree of freedom is-
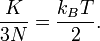
(5)
As noted in the article on heat capacity, diatomic gases should have 7 degrees of freedom, but the lighter gases act as if they have only 5.
Thus the kinetic energy per kelvin (monatomic ideal gas) is:
- per mole: 12.47 J
- per molecule: 20.7 yJ = 129 μeV.
- per mole: 3406 J
- per molecule: 5.65 zJ = 35.2 meV.

No comments:
Post a Comment